
About us
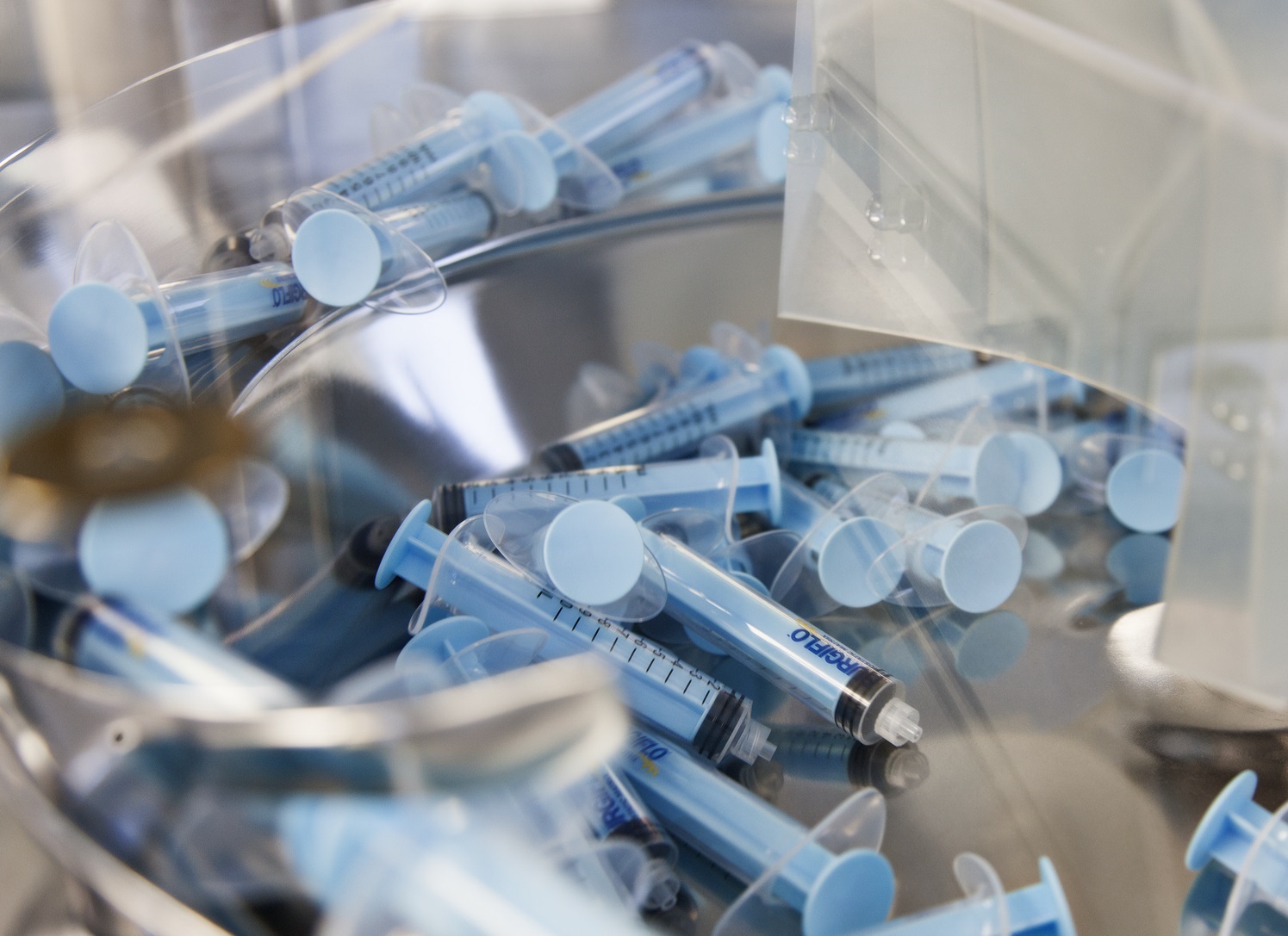
We provide a range of products used by health care professionals in the fields of surgery and diagnostics. Our technology includes Biomaterials used as hemostatic agent for surgery to control bleedings during operations in hospitals. Furthermore, we have strong competences within miniature electromechanics used in devices for regional anesthesia and minimally invasive surgery.
Long time history in development and manufacturing of medical devices for haemostasis
Since 1947 Ferrosan Medical Devices has developed and manufactured haemostatic products, used by clinical professionals to stop bleedings during operations in hospitals. We work in close partnership with Ethicon (Johnson & Johnson), who is responsible for sales and marketing of our products, sold under the SURGIFLO™, SPONGOSTAN™ and SURGIFOAM® trademarks in more than 100 countries.
We are 240 employees in the headquarter in Soeborg, close to Copenhagen. Our focus is development and manufacturing of products with focus on new, innovative solutions and surgical technique for our customers globally.
Ferrosan Medical Devices operates in two main divisions; Biomaterials & Electromechanics division. The majority of the company operates within Biomaterials that develop and manufacturer adjunctive haemostatatic products.
With a revenue of app. DKK 600m in 2020 we have been experiencing strong growth in more ways than one. First of all, due to the global success of our products, as a rapidly increasing international sales are showing healthy growth. Secondly, with a strong focus on innovation and a sound pipeline of new development projects combined with strong investments in optimizing the existing business. Our company has seen a significant growth in headcount and at the present time.
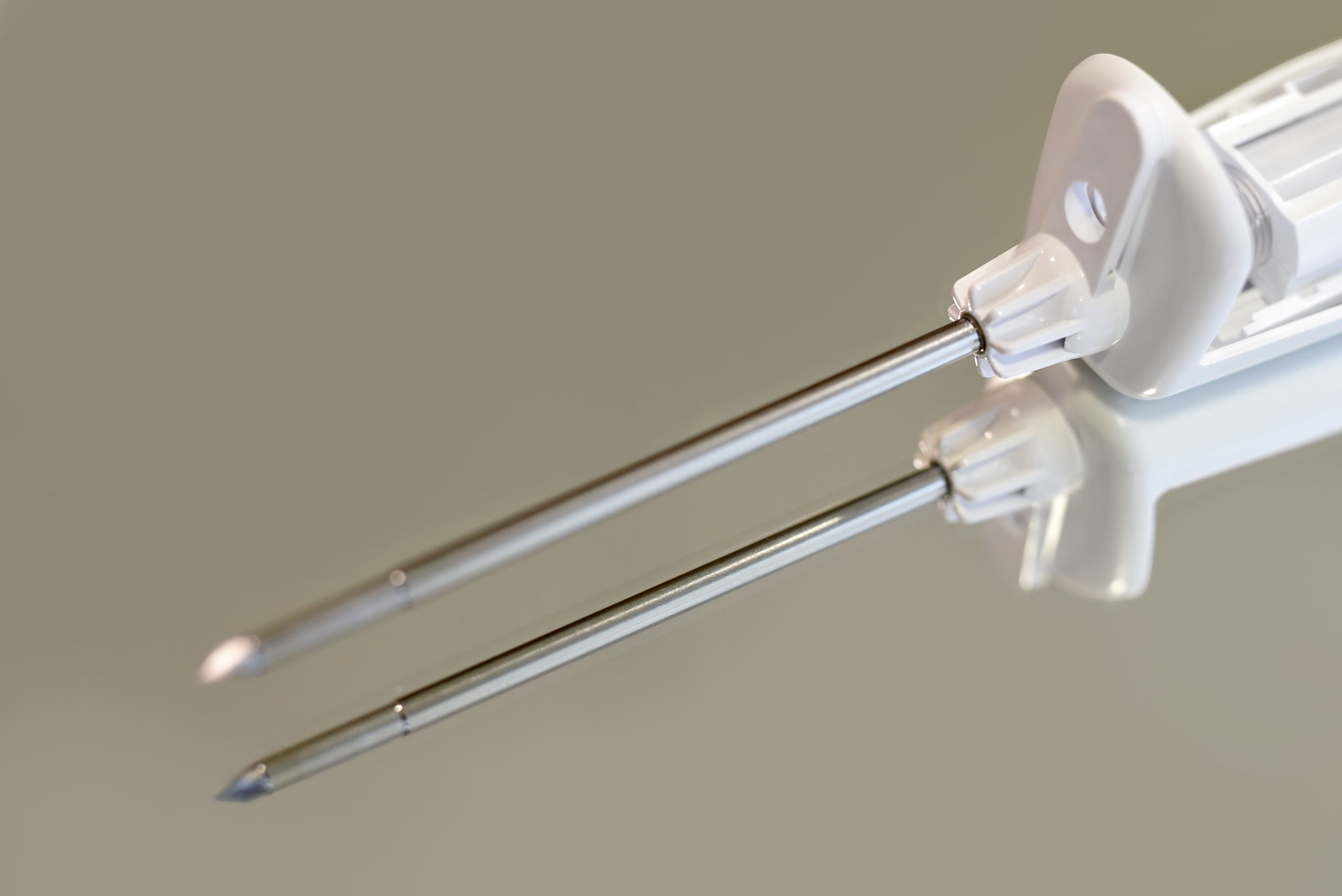
Ferrosan Medical Devices – Electromechanics division additionally employs 100 employees, primarily in Szczecin, Poland that develop and produce handheld biopsy instruments, used by physicians for breast cancer diagnostics. In addition, this division also produces electronic pumps for continuous dispensing of medicines.




Heritage as part of the future
In 2010 Ferrosan Medical Devices A/S was demerged from Ferrosan A/S and established its own legal entity. Ferrosan A/S’s engagement in the haemostatic area dates back to 1947 when the company developed and launched its first product, the absorbable gelatin sponge SPONGOSTAN™. The absorbable gelatin sponge was CE approved as a medical device in 1996 and FDA PMA approved in 1999.
It is the extensive knowledge of more than 70 years that is the heritage of the company upon which the future is being built with the development of advanced haemostatic agent products. Today the company is a global provider of gelatin-based topical absorbable haemostatic agent products.
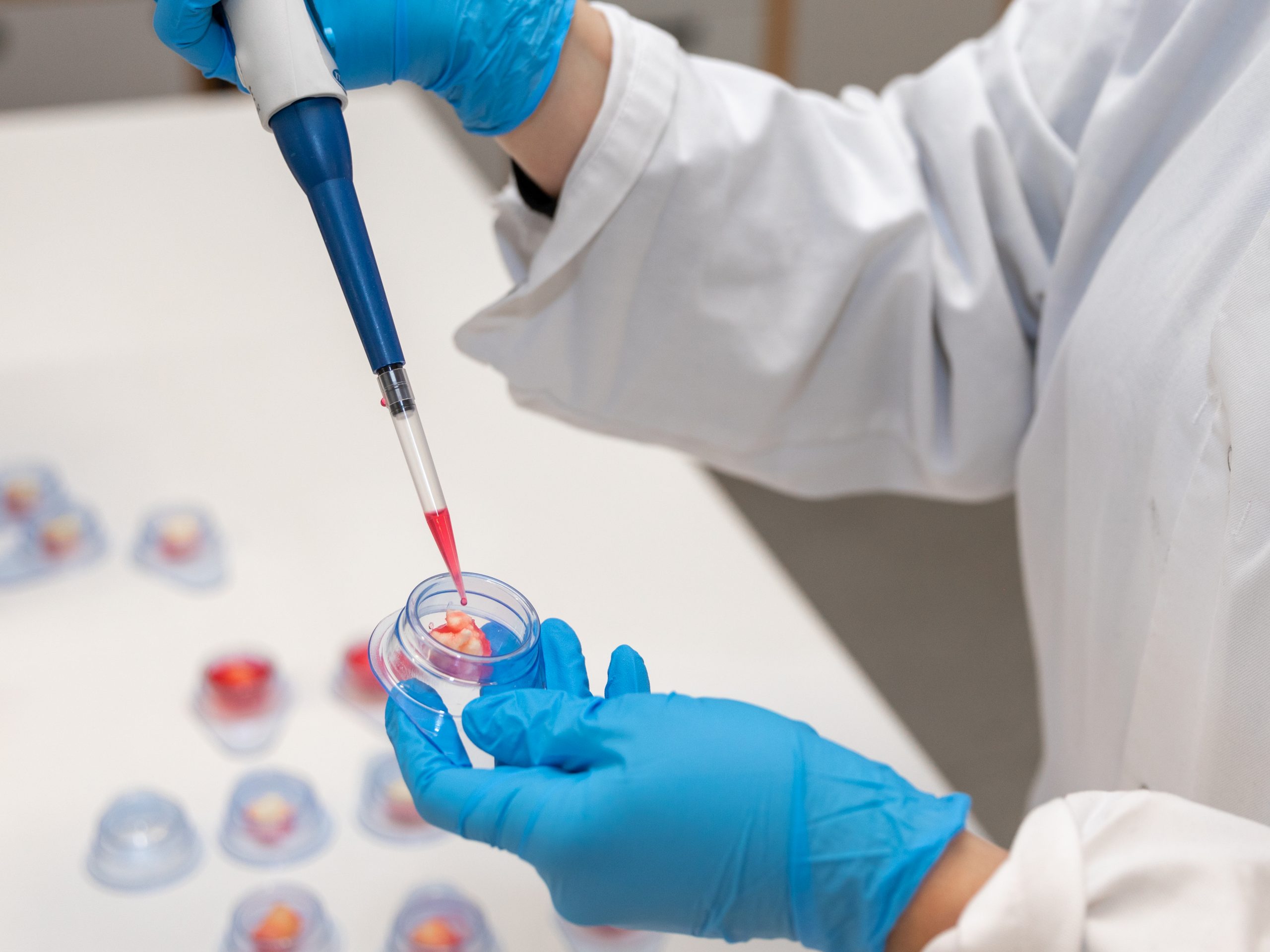
Ferrosan Medical Devices A/S has strong capabilities within clinical insights, research and development as well as operations. As an innovation and manufacturing house, we focus on these activities within the value chain. We believe that this is where we best utilize our capabilities to provide added value to the surgeons and nurses who use our products in critical surgical settings.
Our headquarters are located in Soeborg, close to Copenhagen, where we have production, laboratories, administration, business development, research & development, engineering, regulatory affairs, quality assurance and supply chain all within walking distance of one another.

We provide haemostat solutions that add value to professional health care providers
Vision
Become a major contributor (within our chosen fields) to improving patient safety and surgical outcome
Mission
We develop and manufacture biomaterial based medical devices, preferred by clinicians for the handling of critical challenges in surgical procedures.
We do this by:
- Truly understanding the surgical procedures, the needs and requirements of the health care professionals and ultimately the patients
- Innovating comprehensive solutions meeting the needs and requirements better than any
- Manufacturing uniform high-quality products and delivering dependently
- Acting with conscience towards society and our stakeholders
- Attracting highly committed and competent employees dedicated to pursue our vision
Thus, adding value to our partnership with Ethicon (a Johnson & Johnson company)
GDPR - how Ferrosan Medical Devices A/S handles personal data
Ferrosan Medical Devices A/S handles personal data in accordance with applicable data protection laws, and it is a priority to handle personal data with integrity and according to the internal policies. Here is how Ferrosan Medical Devices A/S process personal data.
Our history
In 2010 Ferrosan Medical Devices A/S was demerged from Ferrosan A/S and established as its own legal entity. Ferrosan A/S has a long history developing haemostatic products for clinical professionals.
Our Values
We believe that the foundation of our success is our employees. We are a strong team; innovative, open-minded, empowered and focused on creating added-value solutions to our partners.


