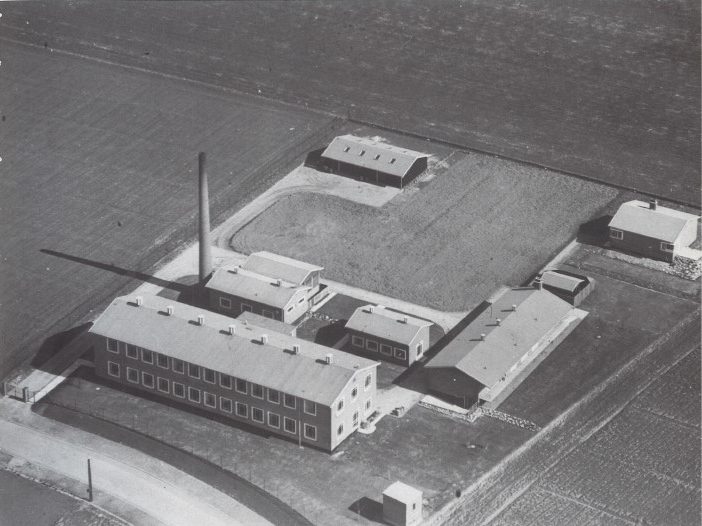
Ferrosan A/S was founded in 1920 in Copenhagen by Niels Jacob Herman Weitzmann. Ferrosan means “healthy iron”. Ferrosan developed the iron supplement products later known as Idozan. Subsequently, the drug Sanocrysin for the treatment of chronic rheumatoid arthritis was launched.
In 2010 Ferrosan Medical Devices A/S was demerged from Ferrosan A/S and established as its own legal entity. The engagement of Ferrosan A/S in the haemostatic area dates back to 1947 when the company developed and launched its first product, the gelatin sponge SPONGOSTAN™.
The gelatin sponge was developed in close collaboration with professor, dr.med. Jens Herman Bing – Professor of General Pathology and Head of the Pathophysiological Laboratory at the University of Copenhagen’s Department of General Pathology. Jens Herman Bing was affiliated with Ferrosan A/S from 1947.
The gelatin sponge was CE approved as a medical device in 1996 and approved by the Food and Drug Administration (FDA) through a pre-market approval (PMA) in 1999.
It is the extensive knowledge of more than 75 years that is the heritage of the company upon which the future is being built with the development of advanced haemostatic agent products. Today, we are a global provider of gelatin-based topical absorbable haemostatic products and handheld biopsy devices.